Understanding the differences between fouling and scaling can help you be in a better position the next time you open your plate heat exchanger. We”ll examine the differences and discuss how each type of resistance to heat transfer can affect the performance of your plate heat exchanger.
Whether you”re running your plate heat exchanger in the basement of a building or in a large industrial complex, you”re likely to run across fouling or scaling (or maybe both) in your heat exchangers at some point. Both fouling and scaling essentially provide an additional (and often times significant) resistance to heat transfer in your heat exchanger. In plate heat exchangers, the degradation of performance is usually magnified because plate exchanger depend on a thin plate wall to maintain their high efficiencies.
Often times, engineers will choose plate heat exchangers because of their ability to mitigate fouling on the surface of the plates. Plate heat exchangers that are designed for a reasonable pressure drop (between 10-15 psig) will generally have a strong enough shear rate between the fluid and the plate wall, that particles have a difficult time adhering to the wall of the plate. However, if a plate heat exchanger is poorly designed or is run at flow rates more than 20% below their intended design, then this shear rate can be reduced to a point where fouling can certainly occur.

We tend to think of fouling as any particles that are able to “cling” to the surface of the plate, but can be removed relatively easily by mechanical means such as hydroblasting or scrubbing with a soft bristle brush. Some types of fouling (such as typical cooling tower fouling) also respond well to caustic cleaning-in-place (CIP) treatments if opening the heat exchanger is not ideal or frequent treatment is needed. An example of typical fouling is shown above. This is an iron-based fouling found in a hot water loop in a commercial building. The fouling came off easily with low pressure water in the field.
Fouling can usually be taken care of during a typical maintenance cycle. Again, either mechanical cleaning or CIP can be utilized, however, CIP does require that isolation valves and chemical taps are provided on the piping. In a given environment, most end users of heat exchangers become familiar with their process fluids and can plan accordingly if they believe that CIP will be needed on their heat exchangers.
Cooling towers are often one of the largest sources of fouling matter in the form of biological fouling. Biological fouling often presents as a dark green to brownish “slime” on the surface of the plate. This biological fouling is best controlled via quality water treatment at the cooling tower. Poorly controlled cooling tower water can cause significant fouling issues in all heat exchangers in the closed loop circuit. Water treatment is vital to controlling this type of fouling.
What About Scaling?
Scaling is a very different phenomenon from fouling. Scaling occurs when a mineral film coats the entire surface of a heat exchanger. The most common forms of scale are usually from calcium-based salts such as calcium sulfate or calcium carbonate. What”s so special (or problematic) about these calcium-based salts? They belong to a family of salts referred to as “reverse soluble salts”:
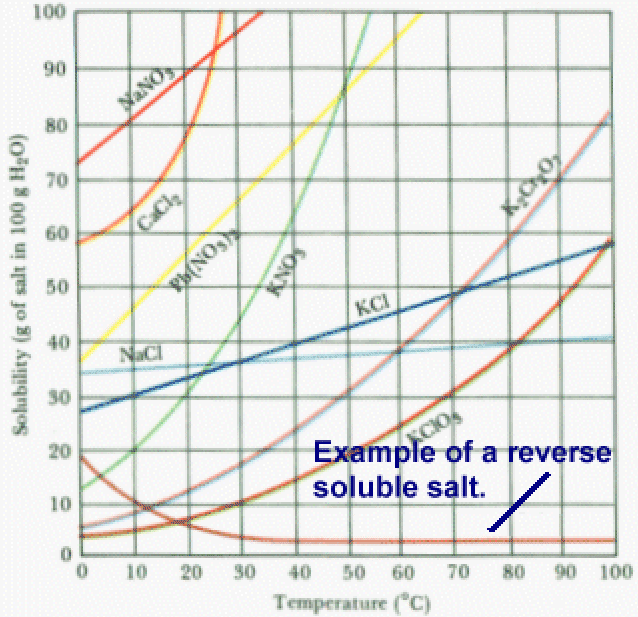
As you can see in the above chart, most salts become more soluble in water at elevated temperatures. This makes perfect sense. Is it easier to dissolve table salt in cold water or hot water? Hot water….sure! But, these reverse soluble salts act in the just the opposite way. As temperature increases, their solubility decreases in water. So what happens when you heat a water-based stream containing these salts is that the salts begin to plate out onto the heat transfer surfaces. Once this happens, the heat transfer performance of your heat exchanger will decrease quickly. For most calcium based salts, this “plating out” effect begins to occur around 140 °F and get even worse at higher temperatures.
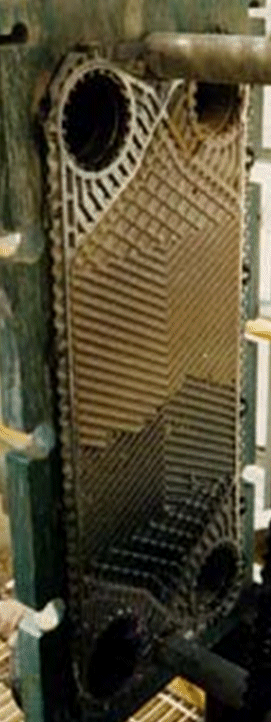
What makes scaling so difficult to deal with is that there nothing that can be done from a design stand point. No amount of shear rate/velocity to stop this from happening! This phenomenon is strictly temperature related. Wait, there”s more bad news. Scale cannot be removed by hydroblasting or your typical scrubbing. The only reliable way to remove these types of scale is by acid cleaning the plates. The scale has to be dissolved and carried away from the plate surface.
The photo above shows what can only be described as a severe case of scaling. This unit is operating in a calcium chloride plant. The natural brine is laden with over 300 ppm of calcium sulfate (gypsum) scale. In this case, the scale not only coats the plate, but continued to build on itself until the channel was plugged. Large chunks of the calcium sulfate could even be removed with a rubber hammer and then the base coating on the plate material removed by acid cleaning.
How Can I Deal with Scaling?
If you suspect scaling in your heat exchanger, the first place to start is with the fluid containing the scaling agent. These mineral scales can come from some boiler treatment and cooling tower chemicals, well waters, or any natural brine. If possible, seek any ways to remove or minimize the scaling agent…first and foremost. Second, investigate any possibilities that may exist to lower the temperature that the stream is being heat to within your process. After that, all we can do is plan to deal with the scaling agent. With regards to plate heat exchangers, the plates can be descaled by a mild acid CIP or by removing the entire plate pack and dipping it in an acid tank.
Be sure to monitor your heat exchanger”s performance over time to pick up on any warning signs that your unit may be suffering from fouling or scaling.



Pingback: Plate Heat Exchangers: Five Questions and Answers – Virginia Heat Transfer